Six thoughts: Temperature-controlled shipping
See how medications are protected in transit.
Introduction
From the polar vortex to summer heat domes, the weather is getting a lot more attention these days. Yet even “ordinary” temperatures play a critical role when shipping certain medicines.
Many specialty medications must be kept within strict temperature ranges in order to arrive at their destinations fully potent and undamaged. This is done by a special shipping process called cold chain logistics. Here are six of the main issues that affect this critical part of the pharmacy delivery system.
1. What is a cold chain?
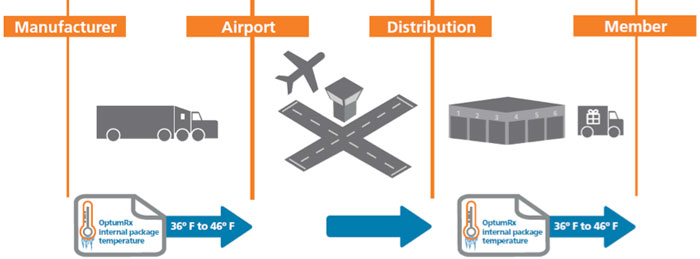
In the pharmacy world, a cold chain is a system of refrigerators, cold storage facilities, and disposable cold boxes. All of these are organized so that temperature-sensitive medications are kept at the right temperature from factory to the point of use.1
A properly functioning cold chain delivery system keeps sensitive medicines within a designated temperature range as they move through the supply system. The US Food and Drug Administration requires that refrigerated biologics be stored and transported within 2°C to 8°C (36°F to 46°F) – unless a medicine is deemed stable at other temperature ranges.2
Typical Cold Chain Distribution
2. Biologic drugs driving increased cold chain demand
The role of the cold chain is becoming more important every day, thanks to the rise of biologic drugs.
Biologic drugs are different from traditional drugs. Where a traditional drug is manufactured from basic chemicals like carbon, sodium, or calcium, biologics are manufactured inside a living system, such as a microorganism, or plant or animal cells. As a result, these drugs are often sensitive to temperature extremes during shipment.4
Biologic drugs that are exposed to excessive heat or cold may put members at risk. Patients who use medicines that arrive with their potency reduced or even destroyed may experience clinical setbacks, recurring symptoms, and other adverse events.5
Recent data show that spending to transport temperature-controlled drugs is growing at roughly double the pace for pharmaceutical products overall.6 This doubling of cold-chain growth is primarily due to the growth of biologic drugs, although insulin products and vaccines (which also require the cold chain) continue their own strong growth.6
And the cold chain will continue to grow to be even more important. Measuring the period between 2014 and 2020, projections are that products that require cold chain transport will rise 65%, while non-cold-chain products may rise by only about half that much.6
3. High AND low temperatures matter
Ultimately, the manufacturer’s label defines how each drug is required to be shipped and stored. The U.S. Food and Drug Administration (FDA) backs these requirements with regulations that define appropriate storage conditions for pharmaceuticals (CFR2111).7
For distributing specialty medications to members, we are most often dealing with refrigerated products that must be held within 36°- 46°F – i.e., cold chain.1
The international shipping industry has specific standards for each step in the cold chain process. Shippers must prove that the inside of each shipping container can maintain the proper temperature range for 24 hours* after they leave the fulfillment center.8
One key is that both the upper and the lower temperature limits are important. It would be relatively simple to pack a medicine with so much coolant that it would be sure to arrive while still cold. But that means it could also possibly be frozen, which can be equally damaging to a biologic drug.
For example, in 2014 Ampio Pharmaceuticals was forced to delay the results of a clinical study on a new biologic drug. They determined that both the study drug and placebo may have frozen during shipment to clinical sites. Unfortunately, the drug in question specifies precise minimum temperature conditions because it may lose potency if it is exposed to temperatures approaching freezing.9
4. Many systems fail
The fact is that controlled temperature shipping is not easy to do. Without careful execution, sensitive items like medicines can be exposed to damage.
As we’ve seen, exposing packages to freezing temperatures can be a problem. But it’s hot weather that continues to dominate the headlines.10, 11
One much-cited study sent dummy packages with embedded temperature sensors to 32 states. They found that more than one in four mail-order prescription deliveries in the US was likely to be exposed to excessive heat while en route to the consumer.12
A different study tested five common packaging technologies used by specialty pharmacies. They applied the standard temperature-control guidelines and subjected the packages to simulated real-world temperatures under laboratory conditions.2
Of the five systems tested, not one maintained the 36°- 46°F temperature range typically required for biologics during the first 24 hours. In fact, two of them didn’t maintain the interior package temperature at all.2
The study goes on to note an important detail: The next 6-hour period (24 to 30 hours) is also critical. This period covers the so-called “last mile,” when medicines can be delayed or left exposed at the last stop in the cold chain (think of mail boxes or front porches).2
As we will see, shippers need to pay special attention to the last mile, since this is where shipments are most vulnerable to temperature fluctuations and potential damage. This attention to detail is especially important during extreme weather events in the winter and summer.
In addition to the clinical risks, improper shipping could also mean that employer plans are unknowingly reimbursing for ineffective drugs. AARP reports that the average cost for specialty drugs for chronic conditions is more than $53,000. On a monthly basis that would be well over $4,000 per shipment.3 Clearly, having to re-send multiple damaged shipments could prove very costly.
5. OptumRx exceeds international shipping standards
The good news for OptumRx clients is that OptumRx ships all temperature-sensitive medications in carefully designed temperature-controlled packaging. We have been working for years to research, benchmark, test, and re-test our packaging systems, with results certified by a third party test laboratory.
As a result, today our cold chain packaging not only meets but exceeds international standards.
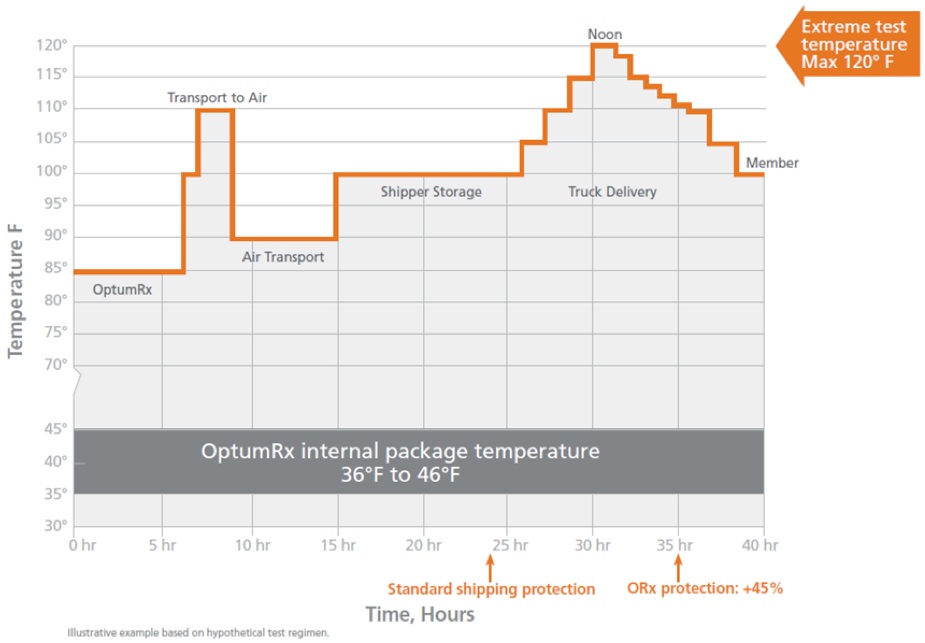
The amount of time a package takes to arrive is a critical variable. While the standard is to protect inside package temperature for 24 hours, OptumRx packaging supports a 35 hour shipping and delivery duration.13 This means our packages provide a full 45 percent more protection than the standard.
That extra time closes the “last mile” gap. Our built-in a 45% time cushion allows for unforeseen delays in the delivery process, plus provides additional hours of protection in case the package must wait at the member’s door.
The second variable is the outside temperature range that the package is expected to travel through.
OptumRx uses different temperature profiles depending on the season. Which profile is used is based on how long the package will be in transit, and more importantly, what part of the country it will travel through on its way to our member. In every case, the profile we choose is based on OptumRx research and testing, which in many cases is more stringent than is standard for the shipping industry.
For example, one common testing protocol (considered the “gold standard”) has a major gap that makes it unacceptable to us. Simply put, the time that the package sits at room temperature while waiting to be picked up by the carrier is not accounted for. OptumRx has incorporated this “dock wait” time into all of its temperature qualification tests.
Protecting against cold
As we discussed above, protecting medications from damage by freezing can be a real challenge, especially when shipping to colder destinations. While the rest of the industry is just starting to realize how important this is, OptumRx has already researched the problem and found a safe shipping solution. Our proprietary method for shipping refrigerated protects medications in the 32°- 0° F temperature range. It covers all but the coldest times of the year.
Protecting against heat
OptumRx uses two profiles to protect against heat damage during shipping: Warm and Extreme Hot/ 120° F.
The OptumRx Warm temperature profile is 60°- 85° F. This is similar to the standard International Safe Transit Association (ISTA) profile called ISTA 7E. This temperature profile can be used in many of the northern, temperate parts of the country, especially outside the peak summer months.
Rising temperatures demand better protection
Extreme Hot 120F
The ISTA 7E standards mentioned above also contain a “severe summer” standard. It covers temperatures between 79°- 91° F. But our real-world data, based on tracking actual packages through the delivery system, shows that even the severe summer standard is not rigorous enough. So we based our hot delivery profile on the hottest day in a hot city such as Phoenix, Arizona, where 110°F is the average recorded temperature in July. Then, to build-in an extra level of protection, we test and certify our packaging to peak temperatures of 120° F.13
Real world testing
Our packaging is designed and tested to show that it can perform reliably under the harshest conditions – all the way from our mail service center to the member. This graph illustrates a representative test routine:
Typical Cold Chain Distribution
6. Ongoing testing for continued improvements
OptumRx has been vigorously pursuing this challenge for years and has developed lightweight, efficient packaging solutions that meet the toughest challenges of the actual distribution system. In addition to controlling temperature, we also take cost, complexity and environmental impacts into account.
For example, we examined the density of the foam shipping boxes. After multiple rounds of tests we found that we could reduce the density of the box by one third, with virtually no measurable difference in temperature control performance.13 That means one third less material used in construction, one third less energy to ship and one third less space in a landfill or other disposal.
Our intensive, persistent cold chain research program is paying off for our customers every day. The best news, of course, is that our carefully tested containers perform where it matters most: protecting fragile medications being shipped in the most extreme temperature conditions during the summer and winter.
Please contact your broker, consultant or your OptumRx representative for more information, or if you have any questions.
* Standard industry definitions allow excursions below and above the specified range as long as the overall effective temperature remains in the specified range.
Pharmacy benefit management
OptumRx pharmacy care services connect the health care system for effective care and better outcomes. Learn more
References
- Logistics Operational Guide (LOG). Cold Chain. Aug 07, 2015. Accessed at: http://dlca.logcluster.org/display/LOG/Cold+Chain on 07.25.2016.
- Modality Solutions. The Cold, Hard Facts: What You Need To Know About Thermal Shipping Technologies. 2013. Accessed at: http://www.modality-solutions.com/a17d423038_sites/modalitysolutions/files/modality_specialty_pharma_whitepaper.pdf on 07.25.2016.
- Medscape Medical News. Specialty Drugs a Focus at HHS Forum on High Drug Costs. Nov. 23, 2015. Accessed at: http://www.medscape.com/viewarticle/854844 on 07.25.2016.
- Biotechnology Industry Association. How do Drugs and Biologics Differ? Accessed at: https://www.bio.org/articles/how-do-drugs-and-biologics-differ on 07.26.2016.
- SpecialtyPharmaJournal.com. Thermal Shipping Technologies – Packing Heat? July 25, 2016.
- Pharmaceutical Commerce. Pharmaceutical cold chain logistics is a $12.6-billion global industry. Updated: April 16, 2016. Accessed at: http://pharmaceuticalcommerce.com/supply-chain-logistics/pharmaceutical-cold-chain-logistics-is-a-12-6-billion-global-industry/ on 07.26.2016.
- Temperature Alert.com. Pharmacy Ambient Temperature and Medication Efficacy. July 14, 2015. Accessed at: http://www.temperaturealert.com/blog/15-07-14/Pharmacy_Ambient_Temperature_and_Medication_Efficacy.aspx on 07.26.2016.
- Pharmaceutical Processing. Cold Chain for Beginners. June 20,2012.
- Pharmaceutical Technology. Packaging Addresses Cold-Chain Requirements. Oct. 02, 2014. Accessed at: http://www.pharmtech.com/packaging-addresses-cold-chain-requirements on 07.26.2016.
- Scientific American. Why the "Heat Dome" Will Scorch Nearly the Entire U.S. This Weekend. July 22, 2016. Accessed at: http://www.scientificamerican.com/article/why-the-heat-dome-will-scorch-nearly-the-entire-u-s-this-weekend/ on 07.26.2016.
- Scientific American. First Half of 2016 Blows Away Temperature Records. July 19, 2016. Accessed at: http://www.scientificamerican.com/article/first-half-of-2016-blows-away-temperature-records/
- Pharmacopeial Forum. Temperature Fluctuations During Mail Order Shipment of Pharmaceutical Articles Using Mean Kinetic Temperature Approach. May-June 1997.
- Internal OptumRx study results. Reported as of August, 2016.
STATEMENT REGARDING FINANCIAL INFLUENCE:
This article is directed solely to its intended audience about important developments affecting the pharmacy benefits business. It is not intended to promote the use of any drug mentioned in the article and neither the author nor OptumRx has accepted any form of compensation for the preparation or distribution of this article.

